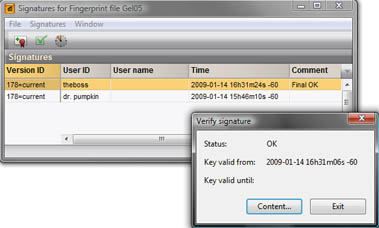
The digital signature tools are useful in environments where data needs to be validated by authorized persons and are fully compatible with FDA 21 CFR Part 11 requirements. Secure digital signature key pairs can be used by authorized users to sign and validate final processed data entries and/or any further changes made. The software can check for the validity of digital signatures and for any fraudulent changes made to the data after digitally signing.